what name is used to refer to the entire d-block elements
Electron arrangements of the d-cake elements
The elements in between Group ii and Group xiii are correctly referred to as the d-cake elements. This is considering the highest energy subshell (i.e. the subshell being filled) is a d-subshell.
Recall that:
- the 3d subshell and the 4s subshell are really very close together in energy, simply 4s is slightly lower in energy, so the 4s fills earlier 3d
- a d-subshell contains five d-orbitals, so can concur upwardly to 10 electrons
- electrons get one into each orbital first, before whatever orbital gets a 2d electron
When we examine the electron arrangements of the kickoff row of d-cake elements we notice two anomalies:

A rather simplistic and inadequate explanation for this is to say that having all the d-orbitals one-half-filled is an especially stable organisation, then Cr has 3d and 4s all one-half filled rather that the expected 3div 4sii arrangement. Mo shows the same effect in Flow 5. In the same fashion information technology can be suggested for Cu that having the 3d orbitals all total is more stable than having the expected 3d9 4s2 system. In other periods, Ag and Au show the same consequence. A more satisfactory explanation is beyond the scope of the A-level course.
Electron arrangements of the ions of d-block elements
Putting electrons into 3d shields the 4s orbital from the nuclear charge, and the 4s electrons now get easier to remove than the 3d i.e. 3d is now the lower energy subshell. Every bit a effect, when nosotros start to remove electrons from the d-block atoms to form ions, we remove the 4s electrons earlier nosotros remove whatever from 3d .
e.m. iron forms greenish Irontwo+ and orangish Fe3+ ions
Fe 1s2 2stwo 2pvi 3stwo 3psix 3dhalf-dozen 4s2
Atomic number 262+ 1stwo 2s2 2p6 3s2 3p6 3d6 – 2 × 4s electrons removed
Atomic number 263+ 1s2 2s2 2p6 3s2 3p6 3dv – 2 × 4s electrons and i × 3d electron removed
e.g. vanadium forms violet 52+ ions and green V3+ ions
Five 1s2 2s2 2p6 3s2 3phalf dozen 3diii 4s2
V2+ 1s2 2s2 2psix 3s2 3p6 3d 3 – two × 4s electrons removed
V3+ 1s2 2s2 2p6 3s2 3p6 3d2 – two × 4s and i × 3d electron removed
Only
Zinc merely forms colourless ii+ ions,
Zn 1sii 2s2 2p6 3s2 3p6 3d10 4stwo
Zn2+ 1s2 2stwo 2p6 3stwo 3phalf-dozen 3d 10 – 2 × 4s electrons removed
Scandium merely forms colourless 3+ ions,
Sc 1s2 2stwo 2phalf-dozen 3s2 3p6 3di 4stwo
Scthree+ 1s2 2stwo 2p6 3sii 3phalf dozen – two × 4s and one × 3d electron removed
We might propose that color in these ions is associated with having a partially-filled d-subshell , and experimentation would provide a concrete basis for this proposition.
Nosotros also have the basis for the definition of a transition element – which is NOT the same as a d-block element :
A transition chemical element is a d-block element that forms at least 1 stable ion with a partially filled d sub-shell. Therefore in Menstruation four, Zn and Sc are d-block elements but NOT transition elements, whereas Ti – Cu are both d-block elements AND transition elements.
Transition element Chemistry
The partially-filled d-subshell in transition elements, and the ease with which electrons can be removed from, or added to these orbitals, gives them their characteristic backdrop:
- forming coloured compounds and complexes
- acting as catalysts
- having compounds with multiple oxidation states
All transition metals form compounds with ions with +2 oxidation number; in nearly cases this is due to losing the ii electrons from the 4s orbital. However, the 3d electrons can too be lost assuasive transition metals to grade stable ions with higher oxidation numbers. This happens because the 3d and 4s energy levels are so shut in energy.
Ions of transition metals besides readily modify from i oxidation land to another, by accepting or donating electrons from the 3d subshell.
An analogy of colour and multiple oxidation states
A solution containing vanadate(Five) ions is yellow, merely when a reducing agent such as zinc is added, the oxidation state tin can be reduced from +5 in steps down to +two with a corresponding alter in color at each stage:
VO2 + (aq) Oxidation state: +5 Color: xanthous
VO2+ (aq) Oxidation state: +4 Colour: blue *
* ordinarily an intermediate dark-green solution is seen containing a mixture of VOii + and VOtwo+ ions before the blue appears.
53+ (aq) Oxidation country: +three Color: green
V2+ (aq) Oxidation land: +two Colour: lilac
In each stage, the vanadium has been progressively reduced. At the same time, the zinc has been oxidized ( Zn(s) → Zn2+ (aq) ) and then this is a series of redox reactions.
Catalytic beliefs in transition metallic chemical science
Catalysts provide an culling reaction pathway with lower activation energy. We classify catalysts as homogeneous (in the same physical state equally the reactants) or heterogeneous (in a dissimilar physical country to the reactants).
Examples of heterogeneous catalysis
- Iron (solid) is used as the catalyst for the Haber procedure: Northward2(one thousand) + 3H2(yard) ⇌ 2NHthree(g)
- Solid vanadium(Five) oxide, V2Ov, is used as the catalyst for the stride in the Contact process to manufacture sulphuric acid that oxidises sulphur dioxide to sulphur trioxide: SO2(g) + ½Oii(g) ⇌ So3(g)
- Nickel is used in the catalyst in the hydrogenation of margarines, saturating (some of) the alkene functional groups in polyunsaturated vegetable oils: -CH=CH- + H2 → -CH2-CH2–
- Manganese(Four) oxide, MnOii, is used to catalyse the decomposition of hydrogen peroxide into oxygen and water: H2O2(aq) → H2O(l) + ½O2(yard)
The reaction between solid zinc and acids (producing a zinc salt and hydrogen) is catalyzed past the presence of copper(II) ions in the solution. The copper(2) ions react with the surface of the solid zinc in a deportation reaction, forming copper metal. The copper metal and so acts as the goad for the reaction between the zinc and the acid.
Examples of homogeneous catalysis
In solution, transition element compounds can provide an culling reaction pathway for redox reactions by reacting with one reactant, then being regenerated in the reaction with the other reactant. e.grand. if A reacts with B in a redox reaction, with A being oxidised and B reduced, the culling pathway might consist of A existence oxidized by the catalyst, which is itself reduced, and then the catalyst reducing B, and being oxidized back to its original course.
- Hydrogen peroxide oxidises tartrate ions to carbon dioxide gas and methanoate ions. The reaction proceeds very slowly if there is no catalyst, even at elevated temperatures.
 The presence of cobalt(Two) ions in solution catalyses the reaction. The hydrogen peroxide initially oxidises the pink Coii+, to greenish Co3+, and is reduced to water. The cobalt(Three) ion causes oxidiation of the tartarate ion, and equally a result of which the Co3+ is reduced back to Cotwo+ and the pinkish colour returns.
The presence of cobalt(Two) ions in solution catalyses the reaction. The hydrogen peroxide initially oxidises the pink Coii+, to greenish Co3+, and is reduced to water. The cobalt(Three) ion causes oxidiation of the tartarate ion, and equally a result of which the Co3+ is reduced back to Cotwo+ and the pinkish colour returns.
- The reaction between iodide ions, I–, and peroxydisulphate ions, Due southtwoOviii 2- forms sulphate ions and iodine: 2I– (aq) + SiiO8 2- (aq) →2SO4 two- (aq) + Itwo(aq)
This germination of iodine can be highlighted by the improver of starch, which forms a blue-black complex with the iodine. The reaction is catalyzed by the presence of Fe2+ (aq), and when a small amount of this is added the blueish-blackness colour forms much more than chop-chop, with the Iron2+ ions left unchanged at the finish of the reaction.
Step 1:S2Oeight 2- (aq) + 2Fe2+ (aq) → 2SOiv 2- (aq) + 2Fe3+ (aq) reaction involving catalyst
Step ii:2I– (aq) + 2Fe3+ (aq) → I2(aq) + 2Fe2+ (aq) regeneration of catalyst
Complexes and Colour
The solid compounds and aqueous ions of transition metals accept feature colours:
- Cutwo+ (aq) ions are blue
- Co2+ (aq) ions are pinkish
- Iron2+ (aq) ions are pale dark-green
- Fethree+ (aq) ions are yellow-orange
- Mn2+ (aq) ions are pale pink
By contrast, compounds of Zn and Sc are white (colourless in solution). This is because Znii+ ions have a 3d10 electron organization, and Sciii+ have no 3d electrons, so neither has the incompletely filled d-orbitals necessary for colour in transition metallic compounds.
Nonetheless, consider copper(II) sulphate. It is blue when hydrated crystals or in solution, but white when anhydrous. The copper ions are [Ar]3d9 in every case. Nosotros must conclude that the unfilled d-subshell is only ane of the necessary requirements for colour.
When in solution and in the hydrated crystal lattice, the transition metal ions are not merely dissolved in h2o, but they are actually bonded to a number of water molecules, past dative covalent bonds formed from a alone pair on water's oxygen atom to the metallic ion.
e.k. the blue Cuii+ (aq) ion is actually[Cu(HiiO)half dozen]2+ (aq) 
This is an case of a circuitous . Because information technology has an overall accuse, we call it a complex ion .
Because transition metallic ions are small-scale they accept a stiff electric field around them that attracts electron-rich species. Species that have a lonely pair bachelor to donate can form dative covalent bonds to the transition metal ion. We phone call such species ligands .
A ligand is a molecule or ion that tin can donate a lone pair to form a dative covalent bail to a transition metallic ion.
It is the presence of these ligands around the cardinal transition element ion that is the other necessary requirement for colour.
Examples of ligands:
- water HtwoO:
- ammonia :NH3
- chloride :Cl–
- cyanide :CN–
- thiocyanate :SCN–
- hydroxide :OH–
The dative covalent bonds around the transition element ion repel one some other, and so are bundled around the transition element ion in geometric arrangements positioning them as far apart as possible (similar in VSEPR Theory). The number of dative covalent bonds arranged around a transition metal is chosen the co-ordination number.
- Circuitous ions with a co-ordination number of 6 tend to exist octahedral
- Complex ions with a co-ordination number of 4 are tetrahedral or occasionally square planar
Drawing complexes, and writing formulae
Formulae for circuitous ions are written in square brackets with the overall charge on the brackets. No individual charges on ligands or the metallic ion are shown. Ligands are in brackets unless single atoms, with the number of each ligand after the bracket if more than one.
Complex ions are drawn in 3D using shaded and wedge bonds to correspond bonds into and out of the plane of the newspaper. The bonds between ligands and the transition metal ion are dative covalent bonds, so we sometimes use an pointer to indicate the direction in which the electron pair has been donated (towards the transition metal ion), but this is optional.
Equally with the formula, square brackets are used and the overall charge written at the top right.
Examples:
1) [Cu(H2O)6] 2+ octahedral
pale blue Name: hexa aqua copper II ion
Aqueous solutions of all the transition element ions (with no other ligands present) have a co-ordination number of 6 and are octahedral.
ii) [CuClfour]ii- tetrahedral
yellow Name: tetra chloro cuprate II ion
Information technology is the size of the ligands that has the almost significant effect on the geometry of the complex ions – chloride ions are much larger than water molecules, so just four chloride ions will pack around the small transition metal ion, where six water molecules would otherwise fit.
iii) [Atomic number 26(HiiO)5SCN]2+ octahedral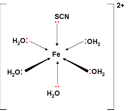
claret carmine Name: penta aqua thiocyanato atomic number 26 III ion
The ligands don't all have to be the same, here five h2o ligands and a thiocyanate ligand form dative bonds to the fe(Three) ion. Observe too that the overall charge on the circuitous ion is two+ even though the iron is in +3 oxidation state, because of the 1- accuse on the SCN– ion.
Crystallising complexes
Complex ions are not constitute on their own – at that place is always an oppositely-charged ion to rest the charges although we may ignore it much of the time. We may focus on the [Cu(HiiO)6]2+ ion when nosotros dissolve copper(II) sulphate in water, forgetting the SO4 2- ion is likewise present in equal concentrations. Similarly when we form [CuCl4]2- past the addition of muriatic acid to copper sulphate solution, we have ii H+ ions present for every [CuCl4]2- ion.
This is of no consequence when nosotros are considering solutions – the "other" ions are spectators, just when we evaporate the water and form crystals, these other ions get function of the behemothic ionic lattice along with the complex ions.
e.grand. Chiliad3Fe(CN)6 contains the complex ion [Fe(CN)6]3- and three K+ ions to balance the charges. Proper noun: potassium hexacyanoferrate(III)
Typical ions we may observe in this office include Na+, K+ or NH4 + . These other ions are NOT ligands and are NOT bonded to the central metal ion.
Ligand Substitution
When a new ligand is added to a solution containing a circuitous ion, the new ligand can supercede an original ligand to form a different circuitous. This is known as ligand exchange .
 Examples:
Examples:
1) Addition of excess full-bodied ammonia solution to Cu2+ (aq) results in the formation a solution containing a dark blue complex ion. Both complexes are octahedral.
[Cu(HiiO)6]two+ + 4NH3 → [Cu(NHthree)4(H2O)2]ii+ + 2HiiO
ii) If full-bodied hydrochloric acid is added to a solution
containing Cu2+ (aq) , the solution turns light-green and progressively more yellow as chloride ligands replace the water molecules. The new circuitous is yellow, but the solution containing both complexes appears light-green.
[Cu(HtwoO)6]2+ + 4Cl– ⇌ [CuCl4]two- + 6HtwoO
Multidentate ligands
All the ligands we take seen so far have formed a single dative covalent bail to the transition element ion. We refer to these as monodentate (literally: 1-toothed) ligands.
Larger molecules may accept more than i site that is able to donate a lone pair, and so may be able to form more than one dative covalent bail to the transition element ion.
Examples: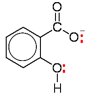
salicylate (2-hydroxybenzoate) ion
Salicylic acid (a benzene ring with an adjacent –OH and –COOH group) does non deed equally a ligand, but the salicylate ion does (presumably because information technology is more than electron-rich).
The O of the –OH group and the –O– of the –COO– group each have a alone pair which can class a bond to the transition metal ion, so ii dative covalent bonds are formed. This equally a bidentate ligand.
When salicylate ions are added to a solution containing yellow aqueous atomic number 26(III) ions, the six water ligands are replaced with three salicylate ligands to form a deep purple circuitous of iron(III) salicylate. (Note that this complex has no accuse – information technology is non a complex ion, just a complex) .
[Fe(HiiO)6]3+ (aq) + 3HOC6HfourCOO– (aq) → [Fe(HOChalf-dozenHivCOO)3](southward) + 6H2O(l)
EDTA (ethylenediaminetetraacetic acrid)
This ligand exists in complexes equally the EDTA4- ion. It has six lone pairs which tin can form
dative covalent bonds, and is hence a hexadentate ligand.
EDTA is used to bind metal ions, removing them from solution, and is referred to equally a chelating agent. Uses include binding calcium and magnesium ions to reduce water hardness, or being added to blood to treat patients suffering from lead or mercury poisoning.
ethane-ane,2-diamine NH2CHtwoCHiiNH 2
This has two N atoms each with a lone pair that can form a bond to the transition metal ion. This is therefore a bidentate ligand.
For instance, nickel forms an octahedral complex with co-ordination number 6 when information technology bonds with three of these ligands:[Ni(NHtwoCHiiCH2NHtwo)3]2+
ethanedioate ions CtwoO4 2- 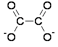
The ethanedioate ion is a bidentate ligand. Each O– can form a dative covalent bail to the transition metal ion.
Cr3+ forms an octahedral complex with ii  ethanedioate ions and ii water molecules: [Cr(CtwoO4)2(HtwoO)2]–
ethanedioate ions and ii water molecules: [Cr(CtwoO4)2(HtwoO)2]–
Nosotros might be given the structure of a complex and asked to piece of work out and draw the ligands nowadays, indicating how they human action as ligands. To do this we need to break the dative covalent bonds to the transition element ion and restore the solitary pair to the atoms in the ligand that formed the bond:
due east.grand. [Pt(CiiO4)( HOCsixH4OCOO)]–
1) Accept the complex ion apart.

2) Look for atoms whose valency is non satisfied with bonds – i besides few bonds indicates a negative ion.

3) Add in the alone pairs which formed the dative covalent bonds to the metal ions.

Biological role of ligand substitution
Haemaglobin in claret is responsible for the transport of oxygen to cells for respiration, and the transport away of carbon dioxide.

Haemaglobin is a complex protein, containing four not-poly peptide components called haem groups, each of which has an Fe2+ ion at its eye, and iv dative covalent bonds between the iron and four Northward-atoms in the haem structure, and a further dative covalent bond to the protein globin.
 Oxygen can bind to the iron in the haem group every bit a ligand, giving a co-ordination number of 6, in order to be transported. The colour of the circuitous when oxygen is bound every bit a ligand is the rich red of oxygenated blood.
Oxygen can bind to the iron in the haem group every bit a ligand, giving a co-ordination number of 6, in order to be transported. The colour of the circuitous when oxygen is bound every bit a ligand is the rich red of oxygenated blood.
Carbon monoxide can also demark to the iron at the same bounden site every bit the oxygen would, the stability constant for this circuitous is greater (see later) so the bond between the iron and the CO is stronger and much less likely to break. CO volition therefore demark preferentially if both carbon monoxide and oxygen are present in the lungs; the bounden is irreversible, so that haemaglobin becomes useless. Since tobacco smoking produces carbon monoxide, and this is one of the reasons why smokers go short of jiff.
Isomerism in complex ions
Stereoisomerism arises when the ligands can be arranged in different spatial arrangements. In complexes, equally with organic substances, nosotros will see both cis-trans isomerism and optical isomerism.
Cis – Trans isomerism
When a circuitous contains four of one ligand and 2 of another, nosotros tin can have cis and trans isomers.
e.g. cobalt forms an octahedral complex of formula [Co(NHthree)ivCl2]+. The cobalt is in oxidation country +3 here. When salts containing this complex ion were crystallised, information technology was found that a light-green salt and a purple salt both with the same formula could be crystallized. This was clear evidence for two different isomers:

Cis-trans isomerism is as well possible in some complexes with co-ordination number 4 if they have a square-planar configuration.
Nickel forms a foursquare planar circuitous [NiCl2(NH3)2]. This has no overall charge, as the nickel is in the +2 oxidation state.

A similar circuitous of platinum [PtCl2(NHthree)ii] is one of the virtually constructive drugs in chemotherapy for cancer. It is the cis-course of the complex which is biologically agile. The drug is a liquid, usually administered intravenously as a drip, and goes under the name of cis-platin .
The importance of the exact shape and structure of the molecule is emphasized by the fact that the trans-class is ineffective.
The do good of using cis-platin to treat cancer is that information technology works by binding to the Deoxyribonucleic acid of fast-growing cancer cells, changing the DNA structure and preventing them from dividing to reproduce. Chemotherapy has unpleasant side effects, nonetheless, and a new generation of cancer-treatment drugs requiring lower doses and with fewer side effects than cis-platin have been developed, e.g. carboplatin.
Optical isomerism
Optical isomerism arises with octahedral complexes containing multidentate ligands, where these can be arranged effectually the central transition metal atom every bit non-superimposable mirror images. Recall that optical isomers rotate plane-polarised light differently – one isomer will rotate it to the right, and the other to the left. An equal mixture of isomers will take no effect on plane polarized light considering the rotations cancel out.
Examples:
[Ni(en)iii]two+ – a complex with three bidentate ligands

[Co(en)two(HtwoO)two]2+ – a complex with two bidentate ligands and two monodentate ligands. Complexes like this will have cis and trans isomers, just there will exist two cis isomer are optical isomers of each other.

Case studies in Transition Metal chemistry
Reactions of copper compounds
i) Cutwo+ (aq) with sodium hydroxide
Bluish aqueous copper(II) ions react with hydroxide ions to form a blue precipitate of copper(II) hydroxide. The precipitate is insoluble in backlog sodium hydroxide.
Cuii+ (aq) + 2 OH– (aq) → Cu(OH)2(southward)
ii) Cuii+ (aq) with ammonia solution
Remember that ammonia forms an equilibrium reacting with water when it dissolves, NHthree(aq) + H2O(aq) ⇌ NH4 + (aq) + OH– (aq) so ammonia solution is both a source of NHthree and OH– ligands.
Initially it is the OH– ions that react with the aqueous copper(II) ions, forming a blue copper hydroxide precipitate every bit above.
When excess ammonia solution is added, the copper hydroxide precipitate redissolves undergoing a ligand substitution reaction to form a nighttime blue solution containing the octahedral trans-complex of [Cu(NH3)four(HiiO)2]two+
[Cu(H2O)vi]2+ (aq)+ 4NH3(aq) → [Cu(NHiii)4(HiiO)2]two+ (aq) + 4H2O(l)
iii) Cu2+ (aq) with concentrated hydrochloric acid
When concentrated hydrochloric acid is added to blue aqueous copper(2) ions, a yellow solution containing tetrahedral [CuCl4]2- (aq) is formed in a ligand commutation reaction, although a green color is often seen when both the aqueous ions and the new complex ion are present in solution. As this is an equilibrium, the colour of the solution changes as the relative concentrations of chloride ions and water present alter.
[Cu(H2O)vi]two+ (aq)+ 4Cl– (aq) ⇌ [CuClfour]two- (aq) + 6HtwoO(l)
iv) Cu2+ (aq) with iodide ions
Aqueous copper(II) ions react with iodide ions in a redox reaction that forms brown aqueous iodine and a white precipitate of copper(I) iodide. Notation that the copper(I) ion has a 3d10 electron arrangement, so information technology is not surprising with no partially filled d-subshell that it is white.

Copper(I) compounds are unstable in solution. When solid copper(I) oxide reacts with hot dilute sulphuric acid, a brownish precipitate of copper and a blueish solution of copper(2) sulphate are formed in a disproportionation reaction, where Cu(I) ions are oxidised to Cu(II) and reduced to metallic Cu.
Cu2O(s) + HiiTheniv(aq) → CuSO4(aq) + Cu(southward) + H2O(l)
Reactions of iron compounds
i) Atomic number 26ii+ (aq) and Ironiii+ (aq) with sodium hydroxide
Pale greenish iron(II) ions in solution react with sodium hydroxide to class a dirty green precipitate of iron(Two) hydroxide. On continuing the precipitate turns brown at its surface as iron(Two) hydroxide is oxidized to iron(III) hydroxide.
Feii+ (aq) + 2OH– (aq) → Iron(OH)2(south)
Stake yellow iron(3) ions in solution react with sodium hydroxide to form an orange-brownish precipitate of atomic number 26(3) hydroxide.
Atomic number 263+ (aq) + 3OH– (aq) → Atomic number 26(OH)3(s)
Neither precipitate redissolves on addition of excess sodium hydroxide.
ii) Irontwo+ (aq) and Iron3+ (aq) with ammonia solution
It is only the hydroxide ions in the ammonia solution that react with fe(II) and fe(III) ions to form iron(II) hydroxide and iron(III) hydroxide exactly as above. At that place is no further reaction with the ammonia present, and the precipitates do not redissolve.
iii) oxidation of iron(II) with acidified managanate(Vii)
Pale dark-green aqueous iron(Two) ions volition decolourise royal managante(VII) ions in acidic solution in a redox reaction. The iron(Ii) is oxidized to pale yellow aqueous iron(Iii) ions, while the manganate(VII) is reduced to pale pink aqueous manganese(Two) ions.
MnOfour – (aq) + 8H+ (aq) + 5Fe2+ (aq) → 5Fethree+ (aq) + Mntwo+ (aq) + 4H2O(l)
iv) reduction of atomic number 26(Iii) with iodide ions
Pale yellow aqueous iron(III) ions can be reduced to pale green iron(2) ions by redox reaction with colourless aqueous iodide ions. In this reaction iodine is formed in aqueous solution, which is brown, and masks the colour alter of the fe ions.
2Fe3+ (aq) + 2I– (aq) → Iii(aq) + 2Fe2+ (aq)
Reactions of manganese compounds
i) Mn2+ (aq) with sodium hydroxide
Pale pink aqueous manganese(Ii) ions react with sodium hydroxide to form a pale pinky-dark-brown precipitate of manganese(Ii) hydroxide, which darkens on standing in air. The precipitate is does non redissolve or react further on further add-on of sodium hydroxide.
Mn2+ (aq) + 2OH– (aq) → Mn(OH)2(southward)
two) Mntwo+ (aq) with ammonia solution
It is only the hydroxide ions in the ammonia solution that react with manganese(Two) ions to form manganese(Two) hydroxide exactly as above. In that location is no further reaction with the ammonia nowadays, and the precipitates exercise not redissolve.
Reactions of chromium compounds
The colour of the complex [Cr(H2O)6]3+ (aq) is pale royal. This may exist a surprise since nosotros have consistently referred to the colour change when acidified dichromate(Half dozen) ions are used as an oxidizing amanuensis as orange for dichromate(Vi) and green for chromium(III). This is because the chromium(III) ion formed in the presence of sulphuric acid is actually non [Cr(HiiO)six]3+ but [Cr(H2O)5(And so4)]+ and this is the light-green ion we have been referring to as Crthree+ (aq) .
i) Cr3+ (aq) with sodium hydroxide
Stake royal aqueous chromium(III) ions react with sodium hydroxide to class a green-gray precipitate of chromium(3) hydroxide.
Cr3+ (aq) + 3OH– (aq) → Cr(OH)iii(due south)
On addition of excess sodium hydroxide, this precipitate redissolves to course a dark dark-green solution containing the complex ion [Cr(OH)6]3- (aq).
Cr(OH)3(s) + 3OH– (aq) →[Cr(OH)six]iii- (aq)
2) Criii+ (aq) with ammonia solution
Initially it is the OH– ions that react with the aqueous chromium(III) ions, forming a green-gray chromium hydroxide precipitate as above.
When excess ammonia solution is added, the chromium hydroxide precipitate redissolves undergoing a ligand exchange reaction to form a purple solution containing the octahedral complex [Cr(NHiii)half-dozen]3+
[Cr(H2O)six]3+ (aq)+ 6NHthree(aq) → [Cr(NH3)6]3+ (aq) + 6H2O(fifty)
three) oxidation of chromium(Three) with alkaline hydrogen peroxide
Hot alkaline hydrogen peroxide is a powerful oxidizing agent, and reacts in a redox reaction to oxidise aqueous chromium(Three) ions (green or pale purple, depending on the ligands bonded to the chromium) to yellow chromate(Half dozen) ions.
3H2O2(aq) + 2Cr3+ (aq) + ten OH– (aq) → 2CrO4 2- (aq) + 8HiiO(l)
iv) reduction of dichromate(VI) with zinc under acidic weather condition
The dichromate(VI) ion is orange in aqueous solution. It can be reduced in a redox reaction with a reducing agent such every bit zinc metal nether acidic conditions, forming chromium(Three) ions in solution. The chromium(Iii) ion formed is probable to be light-green, as a ligand from the acrid volition course part of the Crthree+ (aq) circuitous.
Cr2Oseven ii- (aq) + 14H+ (aq) + 3Zn(s) → 2Cr3+ (aq) + 3Zn2+ (aq) + 7H2O(l)
If an excess of zinc is used, the chromium(III) ions in solution tin be reduced in a farther redox reaction to pale bluish chromium(Two) ions.
Zn(southward) + 2Criii+ (aq) → Zn2+ (aq) + 2Cr2+ (aq)
Source: https://chemistryclinic.co.uk/d-block-and-transition-elements/
0 Response to "what name is used to refer to the entire d-block elements"
Post a Comment